State-of-the-art production sites
Sites producing only plasma-derived medicinal products
Transforming plasma into medicinal products relies on very advanced techniques and above all on state-of-the-art know-how.
The Les Ulis site, in the Essonne department of France, is where the upstream phases of production are performed, from receipt of the bags of plasma to the intermediate products.
Plasma fractionation begins in the production units of the plant, where the proteins are isolated using several specific techniques requiring a high level of technicality: cryoseparation, precipitation, depth filtration.
From Plasma to Biomedicinal Products
The Lille site, in the North department of France, then takes over where the Les Ulis site leaves off. It is responsible for the later phases of production, from receipt of the intermediate products to filling of the medicinal products into vials.
It is in the Lille plant that the production steps known as protein purifications are carried out, after viral inactivation of any viruses present. Each purification is different and must be adapted to fit the protein concerned: nanofiltration, chromatography, etc.
The Carvin site, in the Pas-de-Calais department of France, is in charge the packaging of medicinal products and is the a warehouse to store the materials needed to produce medicines.
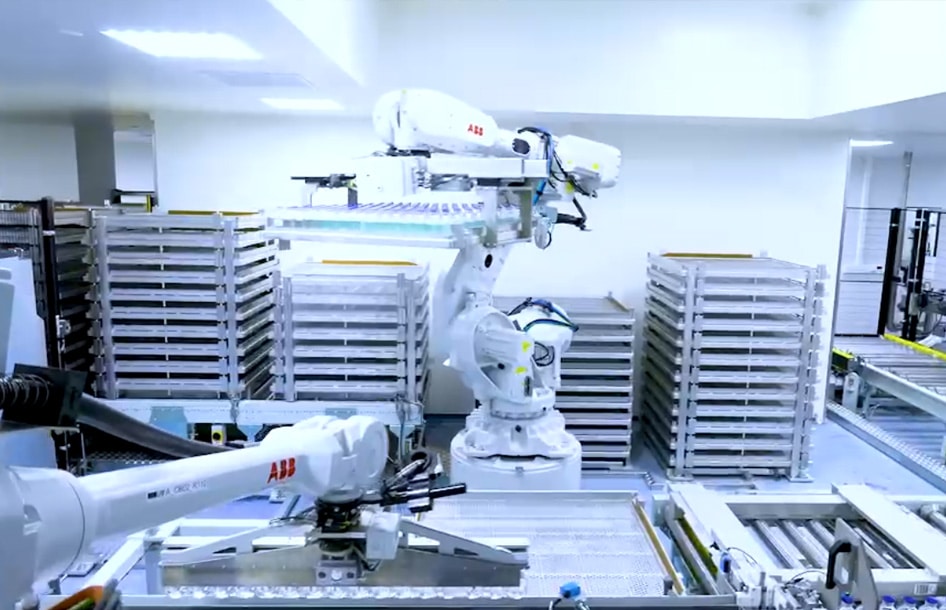
The new generation plant in Arras, commissioned at the end of 2024, centralises in one single site all the cutting-edge production techniques used to produce plasma-derived medicinal products.
The plant is fully automated and operates via gravity, using latest generation industrial equipment based on only the most innovative technologies, all in the service of patients.
Production sites for recombinant medicinal products
LFB is an innovative player in recombinant medicinal products with activities involving the development, manufacturing and marketing of proteins and monoclonal antibodies.
The Alès site plays a key role in LFB’s bioproduction activities.
As a CDMO (Contract Development and Manufacturing Organisation) with US FDA (Food and Drug Administration) approval, the Alès site shares its know-how and equipment with customers, including support for the development of cell lines and processes on an industrial scale, the manufacture of clinical and commercial batches and even the associated analytical testing. As an essential part of LFB’s activities, it is specialised in the bioproduction of therapeutic proteins, and of monoclonal antibodies in particular, by cell culture.
A disruptive technology
Located in the state of Massachusetts in the United States, the Charlton plant specialises in the production of recombinant proteins developed on its rPRO™ technological platform. This technology consists of expressing the desired protein of interest in mammalian milk by genetic recombination.